The program of the main course of organic chemistry and some additional materials demonstrated during lectures - second semester. Aromaticity, criteria for aromaticity. Hückel's rule Aromatic systems criterion for aromaticity
Aromaticity- a concept that characterizes a set of special structural, energetic and magnetic properties, as well as features of the reactivity of cyclic structures with a system of conjugated bonds.
Although aromaticity is one of the most important and most fruitful concepts in chemistry (not just organic), there is no generally accepted short definition of this concept. Aromaticity is understood through a set of special characteristics (criteria) inherent in a number of cyclic conjugated molecules to one degree or another. Some of these criteria are of an experimental, observable nature, but the other part is based on the quantum theory of the structure of molecules. Aromaticity has a quantum nature. It is impossible to explain aromaticity from the standpoint of classical structural theory and resonance theory.
Aromaticity should not be confused with delocalization and conjugation. In the molecules of polyenes (1,3-butadiene, 1,3,5-hexatriene, etc.) there is a clear tendency towards delocalization of electrons and the formation of a single conjugated electronic structure, which is manifested in the spectra (primarily electronic absorption spectra) , some change in bond lengths and orders, energy stabilization, special chemical properties (electrophilic 1,4-addition in the case of dienes, etc.). Delocalization and conjugation are necessary but not sufficient conditions for aromaticity. Aromaticity can be defined as the property in which a conjugated ring of unsaturated bonds exhibits greater stability than would be expected from conjugation alone. However, this definition cannot be used without experimental or calculated data on the stability of the cyclic conjugated molecule.
In order for a molecule to be aromatic, it must contain at least one ring, each of the atoms of which has a p-orbital suitable for the formation of an aromatic system. It is this cycle (ring, system of rings) that is considered aromatic in the full sense of the word (if the criteria listed below are met).
This cycle should be 4n+2(i.e. 2, 6, 10, 14, 18, 22, etc.) p-electrons.
This rule is called a rule or Hückel's aromaticity criterion. The source of this rule is highly simplified quantum chemical calculations of idealized cyclic polyenes made in the early days of quantum chemistry. Further research has shown that this simple rule fundamentally gives correct aromaticity predictions even for very complex real systems.
The rule, however, must be used correctly, otherwise the forecast may be incorrect.
Which orbitals are considered suitable for the formation of an aromatic system? - Any orbitals perpendicular to the plane of the cycle, and
a) belonging to multiple (endocyclic double or triple) bonds included in the cycle;
b) corresponding to lone pairs of electrons in heteroatoms (nitrogen, oxygen, etc.) or carbanions;
c) corresponding to six-electron (sextet) centers, in particular carbocations.
Aromaticity criteria.
Energy(increasing thermodynamic stability due to delocalization of electrons, the so-called delocalization energy - DE).
You can imagine benzene as a derivative of three ethylene molecules and compare the energies of the initial fragments and the final molecule. Each ethylene molecule has 2 p-electrons (6 in total) in molecular orbitals (MO) of the same energy (α + β), and benzene has 6 electrons located in three bonding molecular orbitals, giving a total more negative value of the energy of the system (α and β is less than 0).
The apparent energy advantage is 2β = 36 kcal/mol or 1.56 eV - this is the EER (empirical resonance energy).
The energy criterion is the most inconvenient and unclear of all. The energy values for this criterion are always calculated, because, as a rule, it is impossible to select the corresponding non-aromatic molecule for comparison. Therefore, one should be calm about the fact that there are many different estimates of the delocalization energy even for classical aromatic molecules, but for more complex systems these values are completely absent. You can never compare different aromatic systems based on the magnitude of delocalization energies - you cannot conclude that molecule A is more aromatic than molecule B, because the delocalization energy is greater.
Structural- a very important, if not the most important, criterion, since it is not theoretical, but experimental in nature. The specific geometry of molecules of aromatic compounds lies in the tendency towards a coplanar arrangement of atoms and equalization of bond lengths. In benzene, the alignment of bond lengths is perfect - all six C-C bonds are the same in length. For more complex molecules, the alignment is not perfect, but it is significant. The criterion is taken as a measure of the relative deviation of the lengths of conjugated bonds from the average value. The closer to zero, the better. This quantity can always be analyzed if structural information is available (experimental or from high-quality quantum chemical calculations). The tendency towards coplanarity is determined by the advantage of the parallel arrangement of the axes of atomic p-orbitals for their effective overlap.
Magnetic(the presence of a ring current is a diatropic system, the effect on the chemical shifts of protons outside and inside the ring, examples are benzene and -annulene). The most convenient and accessible criterion, since the 1H NMR spectrum is sufficient to evaluate it. For an accurate determination, theoretical calculations of chemical shifts are used.
Chemical- tendency towards substitution reactions rather than addition reactions. The most obvious criterion that clearly distinguishes the chemistry of aromatic compounds from the chemistry of polyenes. But it doesn't always work. In ionic systems (for example, in the cyclopentadienyl anion or tropylium cation), substitution cannot be observed. Substitution reactions sometimes occur in non-aromatic systems, but aromatic systems are always capable of addition reactions to some extent. Therefore, it is more correct to call the chemical criterion a sign of aromaticity.
Representation of the energy of an aromatic system.
General formula:
E j (orbital energy of level j) = α + m j β
α is the Coulomb integral, the energy of the C2p orbital,
β - resonance integral, interaction energy of 2 atomic orbitals on neighboring atoms
m j = 2сos(2jπ/N), where N is the number of carbon atoms in the cycle.
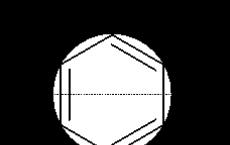
The simplest and most visual graphic representation of energy is frost circle. To construct it, it is necessary to inscribe an aromatic molecule into a circle, pointing its vertex down, then the points of contact of the polygon and the circle will correspond to the energy levels of the MO. An energy scale is applied vertically, all levels below the horizontal diameter are binding, and above are loosening. Electrons are filled from the lowest orbital according to Pauli's rule.

The most favorable state will be when all bonding orbitals are completely filled.
Later, many more assumptions about the structure of benzene appeared:

However, even to this day, the C 6 H 6 molecule continues to present surprises. Bodrikov I.V.: “I have to admit that now there is no person in the world who knows what benzene is” (2009)

(one of the hydrogens moves to a position perpendicular to the ring)
Aromaticity
Aromaticity- a special property of some chemical compounds, due to which the conjugated ring of unsaturated bonds exhibits abnormally high stability; greater than what would be expected with just one pairing.
Aromaticity is not directly related to the smell of organic compounds, and is a concept that characterizes the totality of structural and energetic properties of some cyclic molecules containing a system of conjugated double bonds. The term "aromaticity" was coined because the first members of this class of substances had a pleasant odor.
Aromatic compounds include a wide group of molecules and ions of various structures that correspond to.
Story
Aromaticity criteria
There are several criteria by which a molecule can be classified as aromatic.
Hückel's rule
Molecules that obey Hückel's rule are aromatic: a planar monocyclic conjugated system containing (4n + 2)π-electrons (where n = 0,1,2...) is aromatic. This rule is derived directly from quantum chemical calculations of MOX.
Modern representations
In modern physical organic chemistry, a general formulation of the aromaticity criterion has been developed.
| An unsaturated cyclic or polycyclic diatropic molecule or ion can be considered aromatic if all the atoms of the ring are included in a completely conjugated system in such a way that in the ground state all π-electrons are located only in the bonding molecular orbitals of the annular (closed) shell. |
Aromatic compounds
Aromatization
Aromatization- formation of aromatic compounds from compounds of other types.
The industry widely uses aromatization processes for petroleum products to increase the content of aromatic hydrocarbons in them. The most important is the catalytic reforming of gasoline fractions.
Aromatization processes occur under conditions of biochemical synthesis in plants, animals, fungi and microorganisms. One of the most significant metabolic pathways, of which aromatization reactions are an integral part, is the shikimate pathway.
Sources
- Reutov O.A. Organic chemistry. - M.: Moscow State University Publishing House, 1999. - T. 2. - 624 p. - ISBN 5-211-03491-0
- Agronomov A.E. Selected chapters of organic chemistry. - 2nd. - Moscow: Chemistry, 1990. - 560 p. - ISBN 5-7245-0387-5
- Gorelik M.V. Current state of the problem of aromaticity // Advances in chemistry. - 1990. - T. 59. - No. 2. - P. 197-228.
Notes
| Chemical bond | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| Intermolecular interaction |
| ||||||
1. The molecule has a flat cyclic structure.
2. All atoms in the cycle are in a state of sp2 hybridization (hence the s-skeleton is flat and all sp-orbitals are parallel.
3. In the molecule there is a delocalized p-electron system containing 4n + 2 p-electrons, where n = 0,1,2, is a natural series of numbers. This rule is called Hückel's rule
Heterocyclic compounds also have an aromatic character. When replacing –CH= in a benzene molecule with –N=, the heterocyclic compound pyridine is formed.
Mesomeric effect. Electron-donating and electron-withdrawing substituents. Resonance theory as a qualitative way to describe the delocalization of electron density.
The mesomeric effect or effective conjugation is the transfer of the electronic influence of substituents through a conjugated system. Unlike the I (inductive) effect, the M (mesomeric) effect is transmitted through the conjugation system without attenuation. Deputy lower electr. density in conjugation system (displacement of the ED in its direction) manifested. - M-effect and phenomenon. electron acceptor. (substituents contain multiple bonds of a carbon atom with more negative heteroatoms).
Deputy increased electr. density in conjugation system (displacement of the EF from itself towards the conjugate system) manifested. +M-effect and phenomenon. electron donor. (substituents containing a heteroatom with an unshared pair of electrons)
M-effect (hydroxy, amino, OR, halogens). - M-effect (nitro, sulfo, carboxyl, carbonyl).
Resonance theory- the theory of the electronic structure of chemical compounds, according to which the distribution of electrons in molecules is a combination (resonance) of canonical structures with different configurations of two-electron covalent bonds.
Resonance structures of cyclopentadienide ion
Configuration and conformation are the most important concepts in stereochemistry. Configuration. Elements of symmetry of molecules (axis, plane, center) and operations of symmetry (rotation, reflection). Chiral and achiral molecules. Asymmetric carbon atom as a center of chirality.
Steriochemistry– section of chemistry, study space. built molecules and their influence. on physical and chemical properties, as well as on direction. and the speed of their reaction. It is based on three fundamental concepts: chirality, configuration and conformation.
Configuration– these are spaces. inlet location into the composition of a molecule of atoms or at. groups without taking into account the differences that arose in the following. rotation around single bonds.
Axis of symmetry. If the rotation of a molecule around any axis passing through it is at an angle of 2π/ n= 360°/ n leads to a structure that does not differ from the original one, then such an axis is called the axis of symmetry n-th order C n.
Plane of symmetry (mirror plane) is an imaginary plane that passes through the molecule and divides it into two mirror-like equal parts.
In the presence of center of symmetry all atoms of a molecule that do not lie in the center of symmetry are located in pairs on one straight line passing through the center, at the same distance from the center, as, for example, in benzene.
Conformations molecules - various spatial forms of molecules that arose when the relative orientation of its individual parts changed in the res. internal rotation of atoms or groups of atoms around single bonds, bending of bonds, etc.
If the molecules are incompatible with their mirror image. This property is called chirality, and the molecules themselves – chiral(means that two objects relate to each other as left and right hands (from the Greek. chiros- hand) and are mirror images that do not coincide when trying to combine them in space).
Asymmetric carbon atom - an atom bonded to four different substituents.
Molecules with one center of chirality (enantiomerism). Glyceraldehyde as a configuration standard. Fischer projection formulas. Relative and absolute configuration. D-, L- and R-, S-systems of stereochemical nomenclature. Racemates.
Enantiomers are stereoisomers whose chiral molecules are related to each other as an object and an incompatible mirror image (they represent two optical antipodes and are therefore also called optical isomers).
Glyceraldehyde contains a chiral center, existing in the form of 2 stereoisomers, possessing. various opt.activity.
Projection formulas proposed E. Fischer: 1) carbon skeleton location. vertically; 2) placed at the top. senior function group; 3) the tetrahedron is oriented so that the chiral center is located in the plane, the substituents located to the right and left of the carbon chain are directed forward from the projection plane; Substituents are placed vertically, moving away from the observer beyond the projection plane; The asymmetric carbon atom is transferred to the plane at the intersection point of the horizontal and vertical lines. Relative configuration- this is the relative arrangement of substituents at different asymmetries. atoms in relation to each other; it is usually denoted by prefixes ( cis- And trans-, treo- And erythro- etc.). Absolute configuration- this is the true arrangement in space of substituents at each asymmetric atom of the molecule; most often it is denoted by letters D or L .
R,S-nomenclature.1) Determine the order of precedence of substituents at the chiral center: a) the order of precedence is first established for atoms immediately adjacent. connection with the center: “the higher the atomic number, the older the substituent.” b) if the closest. atoms are the same, then the procedure should be carried out for the atom of the next sphere. 2) Having located the youngest substituent from the observer, determine the direction of decline in the seniority of the remaining three substituents. If it occurs clockwise, it is an R-isomer; if it occurs counterclockwise, it is an S-isomer. D,L-nomenclature(Related to the Fischer projection). If the functional group at the chiral center is on the right, then it is a D-isomer, and on the left is an L-isomer. Enantiomers differ in their ability to rotate plane-polarized light: on the right (+) D, on the left (-) L.
7. The emergence of conformations as a result of rotation around σ bonds. Factors that make rotation difficult. Newman's projection formulas. Types of stress. Energy characteristics of open chain conformations. Relationship between spatial structure and biological activity
1. Conformations (rotational isomerism). Without changing either bond angles or bond lengths, one can imagine many geometric shapes of the ethane molecule, differing from each other in the mutual rotation of carbon tetrahedra around the C-C bond connecting them. As a result of this rotation, rotary isomers (conformers).
In projection Newman the molecule is viewed along the C-C bond). Three lines diverging at an angle of 120° from the center of the circle indicate the bonds of the carbon atom closest to the observer; the lines “poking out” from behind the circle are the bonds of the distant carbon atom.
The conformation shown on the left is called obscured . This name reminds us that the hydrogen atoms of both CH 3 groups are opposite each other. The eclipsed conformation has increased internal energy and is therefore unfavorable. The conformation shown on the right is called inhibited , implying that free rotation around the C-C bond is “inhibited” in this position, i.e. the molecule exists predominantly in this conformation.
The minimum energy required to completely rotate a molecule around a particular bond is called rotation barrier for this connection. The rotation barrier in a molecule like ethane can be expressed in terms of the change in potential energy of the molecule as a function of the change dihedral (torsion) angle systems. The dihedral angle (denoted by ) is shown in the figure below:
As the molecule becomes more complex, the number of possible conformations increases. Below, the conformations of n-butane are depicted as Newman projections. The conformations shown on the left (shaded) are energetically unfavorable; only inhibited ones are practically realized.
Cycloalkanes. Nomenclature. Small cycles. Electronic structure of cyclopropane. Features of the chemical properties of small cycles (addition reactions). Regular cycles. Substitution reactions. Types of stress. Energy difference between cyclohexane conformations (chair, bathtub, half-chair). Axial and equatorial connections. Receipt. Properties
Physical properties. Under normal conditions, the first two members of the series (C 3 - C 4) are gases, (C 5 - C 16) are liquids, starting from C 17 are solids.. Preparation. 1. The main method for obtaining cycloalkanes is the elimination of two halogen atoms from dihaloalkanes:
2. During the catalytic hydrogenation of aromatic hydrocarbons, cyclohexane or its derivatives are formed: t°, P, Ni C 6 H 6 + 3H 2 → C 6 H 12.
Chemical properties. In terms of chemical properties, small and ordinary cycles differ significantly from each other. Cyclopropane and cyclobutane are prone to addition reactions, i.e. similar in this respect to alkenes. Cyclopentane and cyclohexane are close in their chemical behavior to alkanes, since they undergo substitution reactions.1. For example, cyclopropane and cyclobutane are capable of adding bromine (although the reaction is more difficult than with propene or butene):
2. Cyclopropane, cyclobutane and even cyclopentane can add hydrogen, giving the corresponding normal alkanes.
The addition occurs when heated in the presence of a nickel catalyst:
3. Again, only small cycles enter into the addition reaction with hydrogen halides. The addition to cyclopropane homologues occurs according to Markovnikov’s rule:
4. Substitution reactions. Conventional cycles (C 6 and higher) are stable and only undergo radical substitution reactions like alkanes: t ° C 6 H 12 + Br 2 → C 6 H 11 Br + HBr.
5. Dehydrogenation of cyclohexane in the presence of a nickel catalyst leads to the formation of benzene: t ° Ni
C 6 H 12 → C 6 H 6 + 3H 2 .6. When strong oxidizing agents (for example, 50% nitric acid) act on cyclohexane in the presence of a catalyst, adipic (hexanedioic) acid is formed:
Structural features of cycloalkanes and their chemical behavior. Cyclopropane has a flat structure, so the hydrogen atoms of neighboring carbon atoms are located above and below the plane of the cycle in an energetically unfavorable (“obscured”) position. This is one of the reasons for the “tension” of the cycle and its instability.
Conformations of the six-membered ring: a - chair: 6 - bath. Another possible arrangement of atoms for cyclohexane corresponds to the bath conformation, although it is less stable than the chair conformation. It should be noted that in both the chair and bath conformations, the bonds around each carbon atom are in a tetrahedral arrangement. Hence the incomparably greater stability of ordinary cycles compared to small cycles, hence their ability to enter into substitution reactions, but not addition. Cycloalkanes are saturated cyclic hydrocarbons. The simplest representatives of this series: cyclopropane cyclobutane. General formula CnH2n. Structure. Isomerism and nomenclature.Cycloalkanes are saturated cyclic hydrocarbons. The simplest representatives of this series:
Alkenes. Nomenclature. Isomerism. Methods of obtaining. Electrophilic addition reactions, mechanism. Addition of halogens, hydrohalogenation, hydration and the role of acid catalysis. Markovnikov's rule. Concept of radical addition reactions. Oxidation of alkenes (ozonation, epoxidation).
Alkenes- These are not cyclic hydrocarbons, in the molecules of which 2 carbon atoms are in a state of sp 2 hybridization and are connected to each other by a double bond.
The first representative of the homologous series of alkenes is ethene (ethylene) - C 2 H 4. . The homologous series of alkenes has the general formula C n H 2n. A characteristic feature of the structure of alkenes is the presence of a double bond >C=C in the molecule<. Двойная связь образуется при помощи двух пар обобщенных электронов. Углеродные атомы, связанные двойной связью, находятся в состоянии sp²-гибридизации, каждый из них образует три σ-связи, лежащие в одной плоскости под углом 120º.
Alkenes are characterized by structural isomerism: differences in chain branching and in the position of the double bond, as well as spatial isomerism (cis and trans isomers). According to international nomenclature, alkenes are named by numbering the longest chain starting from the end to which the double bond is closest. According to rational nomenclature, they are considered derivatives of ethylene, where one or more hydrogen atoms are replaced by hydrocarbon radicals. For example, let’s name the substance according to the international (IUPAC) nomenclature: CH 3 – C(CH 3) = CH 2 Isobutylene, unsymmetrical dimethylethylene, 2-methylpropene.
AROMATICITY- a combination of certain properties inherent in a large group of compounds, called, respectively, aromatic.
The term “aromaticity” was introduced in 1865 by F. Kekule, who established the structure of benzene and proposed the formula for it:
The name “aromatic” is due to the fact that among benzene derivatives there are compounds with a pleasant odor (for example, nitrobenzene has the smell of almonds).
Kekule drew attention to the fact that the double bonds in benzene and its derivatives differ markedly in properties from the double bonds in most unsaturated compounds. For benzene, addition reactions (for example, of halogens) at double bonds, which in the case of unsaturated compounds occur quite easily, turned out to be extremely difficult.
In addition, it was discovered that ortho-dichlorobenzene (chlorine atoms are located on two adjacent carbon atoms) does not have the isomers that could be expected based on the proposed structural formula for it, where two chlorine atoms are located either at a single or double bond:
As a result, Kekule proposed calling the bonds in benzene oscillatory, that is, vibrating. Over time, this assumption was further developed and improved.
The most typical reactions for benzene are substitution of hydrogen atoms. The study of the chemistry of benzene showed that replacing a hydrogen atom with any group has a certain and, most importantly, predictable effect on the reactivity of the remaining hydrogen atoms.
If a group that withdraws electrons from the nucleus (for example, methyl) is introduced into the benzene ring, then subsequent halogenation leads to substitution in ortho- And pair- position When an electron-donating group (for example, carboxyl) is introduced, the halogen is directed to meta-position:
For a long time, aromaticity was considered to be a set of specified chemical properties, but gradually more accurate characteristics were found based on the structural features of aromatic compounds.
The electronic structure of benzene and related compounds in the modern understanding is as follows. Participate in the formation of double bonds R-electrons of carbon atoms, orbitals (the region of the most probable location of an electron in space) of these electrons have the shape of three-dimensional eights. In the case of benzene, the orbitals overlap, forming ring orbitals on which all R-electrons of the molecule:

As a result, a single closed electron shell appears, and the system acquires high stability. There are no fixed single and double bonds in benzene, all C–C bonds are averaged and equivalent, therefore, a ring symbol placed inside the ring is more often used to indicate aromaticity:
A ring current appears in the resulting cyclic orbitals, which can be detected by special measurements, further indicating the aromaticity of the compound.
Flat cyclic molecules are aromatic, and the number of electrons ( m), combined into a single cyclic system, must comply with Hückel’s rule:
m = 4n + 2 (n = 0, 1, 2, 3...), n– number of natural series
Shown below are the first three representatives of this series of aromatic molecules that follow Hückel's rule: the cyclopropene cation, benzene, and naphthalene.
The expansion of the concept of “aromaticity” made it possible to apply this term to compounds of the non-benzene type, but at the same time possessing a set of structural and chemical characteristics characteristic of benzene derivatives.
In some compounds where the cycle includes O, S or N atoms, for example, in furan, thiophene, pyrrole, as well as in benzene, there is a stable - in accordance with Hückel's rule - six-electron closed system. Four R- electrons (marked in blue in the figure) provide double bonds of the ring, and two s- electrons (marked in red) are given by oxygen, sulfur or nitrogen atoms that have a lone pair of electrons.
Mikhail Levitsky
Aromaticity is a special property of some chemical compounds, due to which the conjugated ring of unsaturated bonds exhibits abnormally high stability; greater than what would be expected with only one conjugation. Aromaticity is not directly related to the smell of organic compounds, and is a concept that characterizes the totality of structural and energetic properties of certain cyclic molecules containing a system of conjugated double bonds. The term "aromaticity" was proposed because the first representatives of this class of substances had a pleasant odor. The most common aromatic compounds contain six carbon atoms in the ring; the ancestor of this series is benzene C 6 H 6 . X-ray diffraction analysis shows that the benzene molecule is flat, and the length of the C-C bonds is 0.139 nm. It follows that all six carbon atoms in benzene are in sp 2-hybrid state, each carbon atom forms σ bonds with two other carbon atoms and one hydrogen atom lying in the same plane, bond angles are 120º. Thus, the σ-skeleton of the benzene molecule is a regular hexagon. Moreover, each carbon atom has a non-hybrid p-orbital located perpendicular to the flat skeleton of the molecule; all six are non-hybrid p-electrons interact with each other, forming π-bonds, not localized in pairs, but combined into a single π-electron cloud. Thus, circular conjugation occurs in the benzene molecule. Graphically, the structure of benzene can be expressed by the following formula:
Circular conjugation gives an energy gain of 154 kJ/mol - this value is conjugation energy - the amount of energy that must be expended to destroy the aromatic system of benzene.
To form a stable aromatic system it is necessary that p-electrons were formally grouped into 3, 5, 7, etc. double bonds; mathematically this is expressed Hückel's rule : cyclic compounds that have a flat structure and contain (4n + 2) electrons in a closed conjugation system, where n is a natural series of numbers, have increased thermodynamic stability.
31 . Electrophilic substitution reactions in benzene (halogenation, nitration, sulfonation, alkylation, acylation). An idea of the mechanism of electrophilic substitution reactions in the aromatic series, σ- and π-complexes.
Halogenation
To introduce a halogen into the aromatic ring, complexes of halogens with Lewis acids are used as reagents. The role of the latter is to polarize the halogen-halogen bond, as a result of which one of the atoms acquires a positive charge, while the other forms a bond with the Lewis acid due to its vacant d-orbitals.

Nitration
Benzene and its homologues are converted into nitro compounds by the action of a nitrating mixture, which consists of concentrated sulfuric and nitric acids (2:1). The nitrating particle (electrophile) is the nitronium cation NO 2 +, the existence of which in the nitrating mixture is proven by the cryoscopic method: measurements of the freezing temperatures of nitric and sulfuric acids and their mixture indicate the presence of four particles in the solution.

Sulfonation
The sulfonation reaction of arenes is believed to occur in oleum under the action of sulfur trioxide, and in sulfuric acid with the participation of the HSO 3 + cation. Sulfur trioxide exhibits electrophilic character due to the polarity of the S–O bonds.

Friedel-Crafts alkylation
One of the ways to obtain benzene homologues is the alkylation reaction. The transformation is named after S. Friedel and J. M. Crafts, who discovered it. As a rule, haloalkanes and aluminum halides are introduced into the reaction as catalysts. It is believed that the catalyst, a Lewis acid, polarizes the C-halogen bond, creating a deficiency of electron density on the carbon atom, i.e. the mechanism is similar to the halogenation reaction

Friedel-Crafts acylation
Similar to the alkylation reaction is the acylation reaction of aromatic compounds. Anhydrides or halides of carboxylic acids are used as reagents; aromatic ketones are the products. The mechanism of this reaction involves the formation of a complex between the acylating reagent and the Lewis acid. As a result, the positive charge on the carbon atom increases incomparably, making it capable of attacking the aromatic compound.

It should be noted that, unlike the alkylation reaction, in this case it is necessary to take an excess of the catalyst relative to the amount of reagents, because the reaction product (ketone) is itself capable of complexation and binds a Lewis acid.
Electrophilic substitution reactions of σ- and π-complexes characteristic of aromatic carbocyclic and heterocyclic systems. As a result of the delocalization of p-electrons in the benzene molecule (and other aromatic systems), the p-electron density is distributed evenly on both sides of the ring. Such shielding of the ring carbon atoms by p-electrons protects them from attack by nucleophilic reagents and, conversely, facilitates the possibility of attack by electrophilic reagents. But unlike the reactions of alkenes with electrophilic reagents, the interaction of aromatic compounds with them does not lead to the formation of addition products, since in this case the aromaticity of the compound would be disrupted and its stability would decrease. Preservation of aromaticity is possible if an electrophilic particle replaces a hydrogen cation. The mechanism of electrophilic substitution reactions is similar to the mechanism of electrophilic addition reactions, since there are general patterns of reactions.
General scheme of the mechanism of electrophilic substitution reactions S E:
The formation of a pi complex is due to the pi bond in the compound, and the sigma complex is formed due to the sigma bond.
Formation of a π-complex. The resulting electrophile X+ (for example, a Br+ ion) attacks the electron-rich benzene ring, forming a π-complex.
Transformation of a π-complex into a σ-complex. The electrophile takes 2 electrons from the π-system, forming a σ-bond with one of the carbon atoms of the benzene ring. Difference between pi and sigma bonds: A sigma bond is stronger, a sigma bond is formed by hybrid orbitals. A pi bond is formed by unhybridized pi orbitals. A pi bond is more distant from the centers of the atoms being connected, so it is less strong and easier to break.
32. Aromatic hydrocarbons. The influence of substituents in the benzene ring on the isomeric composition of the products and the reaction rate. Activating and deactivating substituents. Ortho-, para- and meta-orientators. Radical substitution and oxidation reactions in the side chain.
An essential feature of the reactions for the production and transformation of aromatic hydrocarbon derivatives is that new substituents enter the benzene ring in certain positions relative to existing substituents. The patterns that determine the direction of substitution reactions in the benzene ring are called orientation rules.
The reactivity of a particular carbon atom in the benzene ring is determined by the following factors: 1) the position and nature of the existing substituents, 2) the nature of the active agent, 3) the reaction conditions. The first two factors have a decisive influence.
Substituents on the benzene ring can be divided into two groups.
Electron donors (of the first kind) are groups of atoms capable of donating electrons. These include OH, OR, RCOO, SH, SR, NH 2, NHR, NR 2, NHCOR, -N=N-, CH 3, CH 2 R, CR 3, F, CI, Br, I.
Electron-withdrawing substituents (of the second kind) are atomic groups capable of withdrawing and accepting electrons from the benzene nucleus. These include S0 3 H, N0 2, CHO, COR, COOH, COOR, CN, CC1 3, etc.
Polar reagents acting on aromatic compounds can be divided into two groups: electrophilic and nucleophilic. The most common processes for aromatic compounds are alkylation, halogenation, sulfonation and nitration. These processes occur during the interaction of aromatic compounds with electrophilic reagents. Reactions with nucleophilic reagents (NaOH, NH 2 Na, etc.), for example, hydroxylation and amination reactions, are also known.
Substituents of the first kind facilitate reactions with electrophilic reagents, and they orient the new substituent in ortho- And pair- provisions.
Substituents of the second kind complicate reactions with electrophilic reagents: they orient the new substituent to the meta position. At the same time, these substituents facilitate reactions with nucleophilic reagents.
Let us consider examples of reactions with different orienting effects of substituents.
1. Deputy of the first kind; electrophilic reagent. The reaction-facilitating effect of the substituent, o-, p-orientation:

2. Deputy of the second kind; electrophilic reagent. The action of a substituent that hinders the reaction; m-orientation:

3. Deputy of the first kind; nucleophilic reagent; m-orientation. Obstructive action of the deputy. Examples of such reactions with an indisputable mechanism are unknown.
4. Deputy of the second kind; nucleophilic reagent, o-, p-orientation:
Orientation rules for electrophilic substitution in the benzene ring are based on the mutual influence of the atoms in the molecule. If in unsubstituted benzene C 6 H 6 the electron density in the ring is distributed evenly, then in substituted benzene C 6 H 5 X, under the influence of substituent X, a redistribution of electrons occurs and areas of increased and decreased electron density appear. This affects the ease and direction of electrophilic substitution reactions. The entry point of a new substituent is determined by the nature of the existing substituent.
Orientation rules
The substituents present on the benzene ring direct the newly introduced group to certain positions, i.e. have an orienting effect.
According to their directing action, all substituents are divided into two groups: orientants of the first kind And orientants of the second kind.
Orientants of the 1st kind ( ortho-para ortho- And pair- provisions. These include electron-donating groups (electronic effects of the groups are indicated in parentheses):
R( +I); -OH( +M,-I); -OR( +M,-I); -NH2( +M,-I); -NR 2 (+M,-I)+M-effect in these groups is stronger than -I-effect.
Orientants of the 1st kind increase the electron density in the benzene ring, especially on the carbon atoms in ortho- And pair-positions, which favors the interaction of these particular atoms with electrophilic reagents. Example:

Orientants of the 1st kind, increasing the electron density in the benzene ring, increase its activity in electrophilic substitution reactions compared to unsubstituted benzene.
A special place among the 1st kind orientants is occupied by halogens, which exhibit electron-withdrawing properties: - F (+M<–I ), -Cl (+M<–I ), -Br (+M<–I ).Being ortho-para-orientants, they slow down electrophilic substitution. Reason - strong –I-the effect of electronegative halogen atoms, which reduces the electron density in the ring.
Orientants of the 2nd kind ( meta-orientators) direct subsequent substitution predominantly to meta-position. These include electron-withdrawing groups:
NO 2 ( –M, –I); -COOH( –M, –I); -CH=O ( –M, –I); -SO3H ( –I); -NH 3 + ( –I); -CCl 3 ( –I).
Orientants of the 2nd kind reduce the electron density in the benzene ring, especially in ortho- And pair- provisions. Therefore, the electrophile attacks carbon atoms not in these positions, but in meta-position where the electron density is slightly higher. Example:

All orientants of the 2nd kind, generally reducing the electron density in the benzene ring, reduce its activity in electrophilic substitution reactions.
Thus, the ease of electrophilic substitution for the compounds (given as examples) decreases in the order:
toluene C 6 H 5 CH 3 > benzene C 6 H 6 > nitrobenzene C 6 H 5 NO 2.
Side chain radical substitution and oxidation reactions
The second most important group of reactions of alkyl aromatic hydrocarbons is free radical substitution side chain hydrogen atom in a-position relative to the aromatic ring.

Preferential substitution in a-position is explained by the high stability of the corresponding alkyl aromatic radicals, and therefore the relatively low strength a-C-H-bonds. For example, the energy of breaking the C-H bond in the side chain of the toluene molecule is 327 kJ/mol - 100 kJ/mol less than the energy of the C-H bond in the methane molecule (427 kJ/mol). This means that the stabilization energy of the benzyl free radical C 6 H 5 -CH 2 · is equal to 100 kJ/mol.
The reason for the high stability of benzyl and other alkyl aromatic radicals with an unpaired electron is a-carbon atom is the possibility of distributing the spin density of the unpaired electron in a non-bonding molecular orbital covering carbon atoms 1", 2, 4 and 6.

As a result of distribution (delocalization), only 4/7 of the spin density of the unpaired electron remains with the non-ring carbon atom, the remaining 3/7 of the spin density is distributed between one pair- and two ortho- carbon atoms of the aromatic nucleus.
Oxidation reactions
Oxidation reactions, depending on the conditions and nature of the oxidizing agent, can proceed in different directions.
molecular oxygen at a temperature of about 100 o C, it oxidizes isopropylbenzene via a radical chain mechanism to a relatively stable hydroperoxide.
33. Condensed aromatic hydrocarbons: naphthalene, anthracene, phenanthrene, benzopyrene. Their structural fragments in natural and biologically active substances (steroids, alkaloids, antibiotics).
Naphthalene - C 10 H 8 solid crystalline substance with a characteristic odor. Insoluble in water, but soluble in benzene, ether, alcohol, chloroform. Naphthalene is similar in chemical properties to benzene: it is easily nitrated, sulfonated, and interacts with halogens. It differs from benzene in that it reacts even more easily. Naphthalene is obtained from coal tar.
Anthracene is colorless crystals, melting point 218° C. Insoluble in water, soluble in acetonitrile and acetone, soluble in benzene when heated. Anthracene is obtained from coal tar. Its chemical properties are similar to naphthalene (it is easily nitrated, sulfonated, etc.), but differs from it in that it more easily enters into addition and oxidation reactions.
Anthracene can photodimerize under the influence of UV radiation. This leads to a significant change in the properties of the substance.
The dimer contains two covalent bonds formed as a result of cycloaddition. The dimer decomposes back into two anthracene molecules when heated or under UV irradiation with a wavelength below 300 nm. Phenanthrene is a tricyclic aromatic hydrocarbon. Phenanthrene appears as shiny, colorless crystals. Insoluble in water, soluble in organic solvents (diethyl ether, benzene, chloroform, methanol, acetic acid). Solutions of phenanthrene glow blue.
Its chemical properties are similar to naphthalene. Benzpyrene, or benzopyrene, is an aromatic compound, a representative of the family of polycyclic hydrocarbons, a substance of the first hazard class.
Formed during the combustion of hydrocarbon liquid, solid and gaseous fuels (to a lesser extent during the combustion of gaseous fuels).
In the environment it accumulates mainly in soil, less in water. It enters plant tissues from the soil and continues its movement further in the food chain, while at each stage the BP content in natural objects increases (see Biomagnification).
It has strong luminescence in the visible part of the spectrum (in concentrated sulfuric acid - A 521 nm (470 nm); F 548 nm (493 nm)), which allows it to be detected in concentrations up to 0.01 ppb by luminescent methods.
34. Halogen derivatives of hydrocarbons. Classification, nomenclature, isomerism.
Halogen derivatives can be classified in several ways:
1. in accordance with the general classification of hydrocarbons (i.e. aliphatic, alicyclic, aromatic, saturated or unsaturated halogen derivatives)
2. by the quantity and quality of halogen atoms
3. according to the type of carbon atom to which the halogen atom is attached: primary, secondary, tertiary halogen derivatives.
According to IUPAC nomenclature, the position and name of the halogen is indicated in the prefix. Numbering begins from the end of the molecule to which the halogen atom is closest. If a double or triple bond is present, then it is this that determines the beginning of the numbering, and not the halogen atom: The so-called “rational nomenclature” for compiling the names of halogen derivatives. In this case, the name is constructed as follows: hydrocarbon radical + halide.
Some halogen derivatives have trivial names, for example, the inhalation anesthetic 1,1,1-trifluoro-2-bromo-2-chloroethane (CF 3 -CBrClH) has the trivial name fluorotane. 3. Isomerism
3.1. Structural isomerism 3.1.1. Isomerism of substituent positions
1-bromobutane 2-bromobutane
3.1.2. Isomerism of the carbon skeleton
![]()
1-chlorobutane 2-methyl-1-chloropropane
3.2. Spatial isomerism
Stereoisomerism can occur when there are four different substituents on one carbon atom (enantiomerism) or when there are different substituents on a double bond, for example:
![]()
trans-1,2-dichloroethene cis-1,2-dichloroethene
35. Reactions of nucleophilic substitution of the halogen atom, their use in the synthesis of organic compounds of various classes (alcohols, ethers and esters, amines, thiols and sulfides, nitroalkanes, nitriles). - makes it possible to obtain representatives of almost all classes of organic compounds (alcohols, ethers, amines, nitriles, etc.), therefore these reactions are widely used in the synthesis of medicinal substances. Basic reaction mechanisms
Substitution of a halogen at an sp 3 -hybrid carbon atom can be carried out by both S N 1 and S N 2 mechanisms. The substitution of the halogen at the sp 2 -hybrid carbon atom (in aryl and vinyl halides) occurs either by the type of addition-elimination or by the type of elimination-addition and is much more difficult than for the sp 3 -hybrid. - S N 1 mechanism includes two stages: a) dissociation of alkyl halide into ions; b) interaction of a cation with a nucleophile Nucleophilic attack of a contact ion pair, in which the asymmetry is largely preserved, leads to a reversal of the configuration. In a solvate-separated ion pair, one side of the cation is shielded by the solvated halide ion and nucleophile attack is more likely on the other side, resulting in preferential configuration reversal, but selectivity is reduced and racemization is increased. Complete racemization is possible only with the formation of a free cation (c). However, complete racemization is not usually observed for optically active halides via the S N 1 mechanism. Racemization ranges from 5 to 20%, therefore, practically no solvated cation is formed.
The stage of carbocation formation is limiting, and, therefore, the stability of the cation determines the speed of the process. The rate of the process also depends on the concentration of the alkyl halide and is independent of the concentration of the nucleophile.
The formation of a carbocation can cause a number of side processes: isomerization of the carbon chain, elimination (EI), etc.
Nucleophile Nu - attacks the substrate from the side opposite to the leaving group. In this case, the reaction proceeds in one stage with the formation of a transition state in which sp 3 -hybridization of the central carbon atom changes to sp 2 - with a p-orbital perpendicular to the plane of location of the hybrid orbitals. One lobe of the etor orbital overlaps with the nucleophile, and the second with the leaving group. The C-Nu bond is formed simultaneously with the cleavage of the C-Y bond.
The rate of conversion of starting substances into reaction products depends on: 1) the magnitude of the positive charge on the carbon atom of the substrate, 2) spatial factors, 3) the strength of the nucleophile and 4) in the kinetic region, the concentration of both the nucleophile and the alkyl halide. With a large excess of nucleophile, the reaction can proceed in the first or fractional order. (The terms S N 1 and S N 2 indicate only molecularity, not the order of the reaction.)
The reaction is always accompanied by a reversal of the configuration. A side reaction may be the elimination of E2.
The S N Ar (addition-elimination) mechanism is usually realized in the presence of electron-withdrawing substituents that create d+ (direct the nucleophile) and stabilize the s-complex. In heterocycles, their role is played by the heteroatom. In contrast to the S N 2 mechanism for alkyl halides, the nucleophile forms a new bond before the old one breaks.
Pyridine and quinoline can be considered as analogues of nitrobenzene. As in nitrobenzene, the position of the halogen in the ring is of great importance. 3-Halopyridines are similar to halobenzenes, 2-,4-substituted ones are similar to nitrohalobenzenes, while 4-halopyridine is more active than 2-substituted. The reactivity of alkyl halides in nucleophilic substitution reactions in protic solvents decreases (the ability of groups to leave decreases) in the following order: RI > RBr > RCl > RF.
In the case of activated haloarenes, the appearance of a positive charge at the reaction center depends not only on the number, location and nature of other substituents in the nucleus, but also on the nature of the replaced halogen. Therefore, halogen atoms can be replaced with increasing ease in row I< Br < Cl < F .Катализ замещения галоген в аренах медью – один из важных технологических приемов, позволяющий ускорить реакцию замещения неактивированного галогена в аренах, снизить температуру реакции (~ на 100 о С), увеличить селективность процесса и выход продукта. Предполагают, что реакция идет через стадию образования медь-органических комплексов
Aromatic substrates (aryl halides) must be activated, otherwise the yield of the target product (ester) may be low due to side processes. The replacement of halogen in primary and secondary alkyl halides with an amino group is carried out by heating them with an alcoholic, aqueous or aqueous-alcoholic solution of ammonia, a primary or secondary amine under pressure (in an autoclave). This produces a mixture of salts of primary, secondary, tertiary amines and quaternary ammonium salts